orbital diagram of helium|The Orbital Diagram of Helium: Understanding the Electron : Bacolod Orbital Filling Diagrams. An orbital filling diagram is the more visual way to . PinayFlix TV; Pinay Porn Videos; Contact Us; Latest videos. Latest videos Longest videos Random videos. HD 04:26. Pinay na mahilig mang thirst trap pumatong kay Ralph. HD 05:11. Nakatikim ng jumbo hatdog si petite pinay. HD 03:22. Ayaw lunukin medyo malansa kasi. HD 03:32. Kinawawa ni Armani ang aking pussy. HD 06:58. Pinadede si damulag.
orbital diagram of helium,Ago 14, 2020 — The electron configuration and orbital diagram of helium are: The n = 1 shell is completely filled in a helium atom. The next atom is the alkali metal lithium with an atomic number of 3.orbital diagram of helium The Orbital Diagram of Helium: Understanding the Electron The simpler wavefunctions for helium atom in Equation \(\ref{5}\), can be interpreted .Orbital Filling Diagrams. An orbital filling diagram is the more visual way to .
Dis 14, 2021 — To write the orbital diagram for the Helium (He) first we need to write the electron configuration for just He. To do that we need to find the number of ele.
orbital diagram of heliumThe electron configuration and orbital diagram of helium are: The n = 1 shell is completely filled in a helium atom. The next atom is the alkali metal lithium with an atomic number of 3.
Ene 30, 2023 — The simpler wavefunctions for helium atom in Equation \(\ref{5}\), can be interpreted as representing two electrons in hydrogen-like 1s orbitals, designated as a 1s 2 .Helium only has 2 electrons and therefore it has a configuration of 1s 2. Because the 1s orbital is full with 2 electrons and any additional electrons would go in a new energy level. The electron .
The Orbital Diagram of Helium: Understanding the Electron May 18, 2021 — Orbital Filling Diagrams. An orbital filling diagram is the more visual way to represent the arrangement of all the electrons in a particular atom. In an orbital filling diagram, .
Now that we have treated the Hydrogen-like atoms in some detail, we now proceed to discuss the next-simplest system: the Helium atom. In this situation, we have two 6z electrons – with .
Learn how to draw and interpret the orbital diagram of helium, a noble gas with two electrons in the 1s orbital. Understand the rules and principles of orbital notation and how they affect the .Helium is composed of two electrons bound by the electromagnetic force to a nucleus containing two protons along with two neutrons, depending on the isotope, held together by the strong .
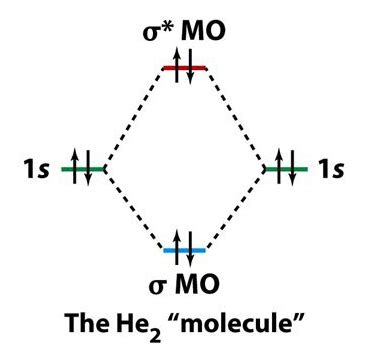
The bonding in any diatomic molecule with two He atoms can be described using the following molecular orbital diagram: . A helium dimer molecule bound by Van der Waals forces was first proposed by John Clarke Slater in 1928 and observed in 1993 by Gentry and coworkers. Interestingly, \(\ce{He_2}\) is the largest known molecule of two atoms .
The electron configuration and orbital diagram of helium are: The n = 1 shell is completely filled in a helium atom. The next atom is the alkali metal lithium with an atomic number of 3. The first two electrons in lithium fill the 1s orbital and .Hul 16, 2022 — In the helium orbital diagram, the 1s subshell accommodates two electrons.. To illustrate the helium orbital diagram, first, determine the number of electrons from the periodic table.Then, note the electron configuration for reference, and follow the three essential rules: Aufbau principle, Pauli exclusion principle, and Hund’s rule.Ene 30, 2023 — Excited States of Helium. The lowest excitated state of helium is represented by the electron configuration 1s 2s.The 1s 2p configuration has higher energy, even though the 2s and 2p orbitals in hydrogen are degenerate, because the 2s penetrates closer to the nucleus, where the potential energy is more negative. When electrons are in different orbitals, their .

The orbital diagram for the helium atom is therefore. written as 1s 2, where the superscript 2 implies the pairing of spins. Otherwise, our configuration would violate the Pauli principle. The next element is lithium, with Z = 3 and three electrons in the neutral atom.Hul 22, 2021 — Molecular Orbital Diagrams. This scheme of bonding and antibonding orbitals is usually depicted by a molecular orbital diagram such as the one shown here for the dihydrogen ion H 2 +.Atomic valence electrons (shown in boxes on the left and right) fill the lower-energy molecular orbitals before the higher ones, just as is the case for atomic orbitals.Khanmigo is now free for all US educators! Plan lessons, develop exit tickets, and so much more with our AI teaching assistant.If you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.Mar 26, 2020 — The electron configuration and orbital diagram of helium are: The n = 1 shell is completely filled in a helium atom. The next atom is the alkali metal lithium with an atomic number of 3. The first two electrons in lithium fill the 1s orbital and have the same sets of four quantum numbers as the two electrons in helium.
Helium atomic orbital and chemical bonding information. There are also tutorials on the first thirty-six elements of the periodic table. Check out the blackboard. . The electrons like to be in separate shells/orbitals. Shell number one can only .Set 20, 2023 — As per the rule of LCAO, the 1s atomic orbitals of two helium atoms overlap to produce two molecular orbitals i.e., a bonding molecular orbital (σ1s) and an antibonding molecular orbital (σ*1s).. On the MO diagram, the bonding MO (σ1s) is placed at a lower energy level, below the 1s AOs of two He-atoms.Nob 26, 2022 — The atomic orbital diagram for the He in the ground state is shown below. Molecular orbital diagram of He in the ground state Conclusion. The article has discussed the electrical configuration of helium, which is 1s 2 and consists of the noble gas’s configuration through the use of this article. Whereas we have also discussed the ground, excited state, and .Ene 28, 2024 — The helium orbital diagram is a graphical representation of the electron configuration of the helium atom. This diagram shows how the electrons in the helium atom are arranged in different orbitals. Orbital is the region of space around the nucleus of an atom where electrons are found.Set 27, 2018 — Molecular Orbital Diagram of Helium Molecule Video Lecture from Chapter Nature of Chemical Bond of Subject Chemistry Class 11 for HSC, IIT JEE, CBSE & NEET.W.

Orbital diagrams depict the distribution of electrons in the various orbitals of an atom’s electron cloud, providing insight into its chemical and physical properties. In an orbital diagram, each orbital is represented by a box, with arrows denoting the electrons. . For example, in the first row of the periodic table, hydrogen and helium .May 18, 2021 — The orbital filling diagrams for hydrogen, helium, and lithium are shown in the figure below. Figure \(\PageIndex{2}\): Orbital filling diagrams for hydrogen, helium, and lithium. According to the Aufbau process, sublevels and orbitals are filled with electrons in order of increasing energy. Since the \(s\) sublevel consists of just one orbital .A helium atom is an atom of the chemical element helium.Helium is composed of two electrons bound by the electromagnetic force to a nucleus containing two protons along with two neutrons, depending on the isotope, held together by the strong force.Unlike for hydrogen, a closed-form solution to the Schrödinger equation for the helium atom has not been found.
orbital diagram of helium|The Orbital Diagram of Helium: Understanding the Electron
PH0 · The Orbital Diagram of Helium: Understanding the Electron Configuration
PH1 · The Orbital Diagram of Helium: Understanding the Electron
PH2 · How to Write the Electron Configuration for Helium
PH3 · How to Write the Atomic Orbital Diagram for Helium (He)
PH4 · Helium orbital diagram
PH5 · Helium atom
PH6 · Electron Configuration for Helium (He)
PH7 · 8: The Helium Atom
PH8 · 8.3: Electron Configurations
PH9 · 6.4 Electronic Structure of Atoms (Electron Configurations)
PH10 · 5.61 F17 Lecture 22: Helium Atom
PH11 · 3.8: Hund's Rule and Orbital Filling Diagrams